Alexey Tomilov, Chase Garcia, Jose Sandoval, Robert Mahley, Gino Cortopassi
Abstract
ApoE4 inheritance is the single largest genetic attributable risk factor for Alzheimer's disease (Mahley, Weisgraber et al. 2006; Yamazaki, Zhao et al. 2019), yet its pathomechanism is unclear, and understanding of its mechanism could lead to targeted therapy (Mahley and Huang 2012). The ApoE3 allele differs from ApoE4 by a single amino acid exchange of Arg to Cys at ORF position 112. ApoE3 arose on a hominid lineage about 200,000 years ago and this derived state could have increased in frequency as the result of a positive selective benefit (Fullerton, Clark et al. 2000). ApoE3 confers increased glucose oxidation in neurons relative to ApoE4 (Chang, ran Ma et al. 2005; Zhao, Liu et al. 2017; Orr, Kim et al. 2019).
Methods: We tested the effect of ApoE alleles in 3 different N2a-based cell models, the ApoE3 and ApoE4 stable transfectants made in the Mahley laboratory (Harris, Brecht et al. 2004), and ApoE2,E3,E4 transiently and stably transfected in neuronal cells in our laboratory. In addition, we investigated the mitochondrial function in hippocampus of ApoE4 mouse model of AD using succinate/rotenone, pyruvate/malate, palmitoylcarnitine/malate, and palmitoylcarnitine/malonate as a substrates.
Results. We investigated the bioenergetic consequences of ApoE alleles in N2a cells from 3 sources, the results were consistent among all 3 data sets.
Finding 1. In the presence of glucose, ApoE3 increased insulin sensitivity, glycolysis, and mitochondrial glucose oxidation relative to ApoE4, ApoE2 and Controls were observed. These results are represented in this poster (Figure 1,2,3), and the posters of Chase Garcia and Jose Sandoval from Cortopassi lab elsewhere in this conference.
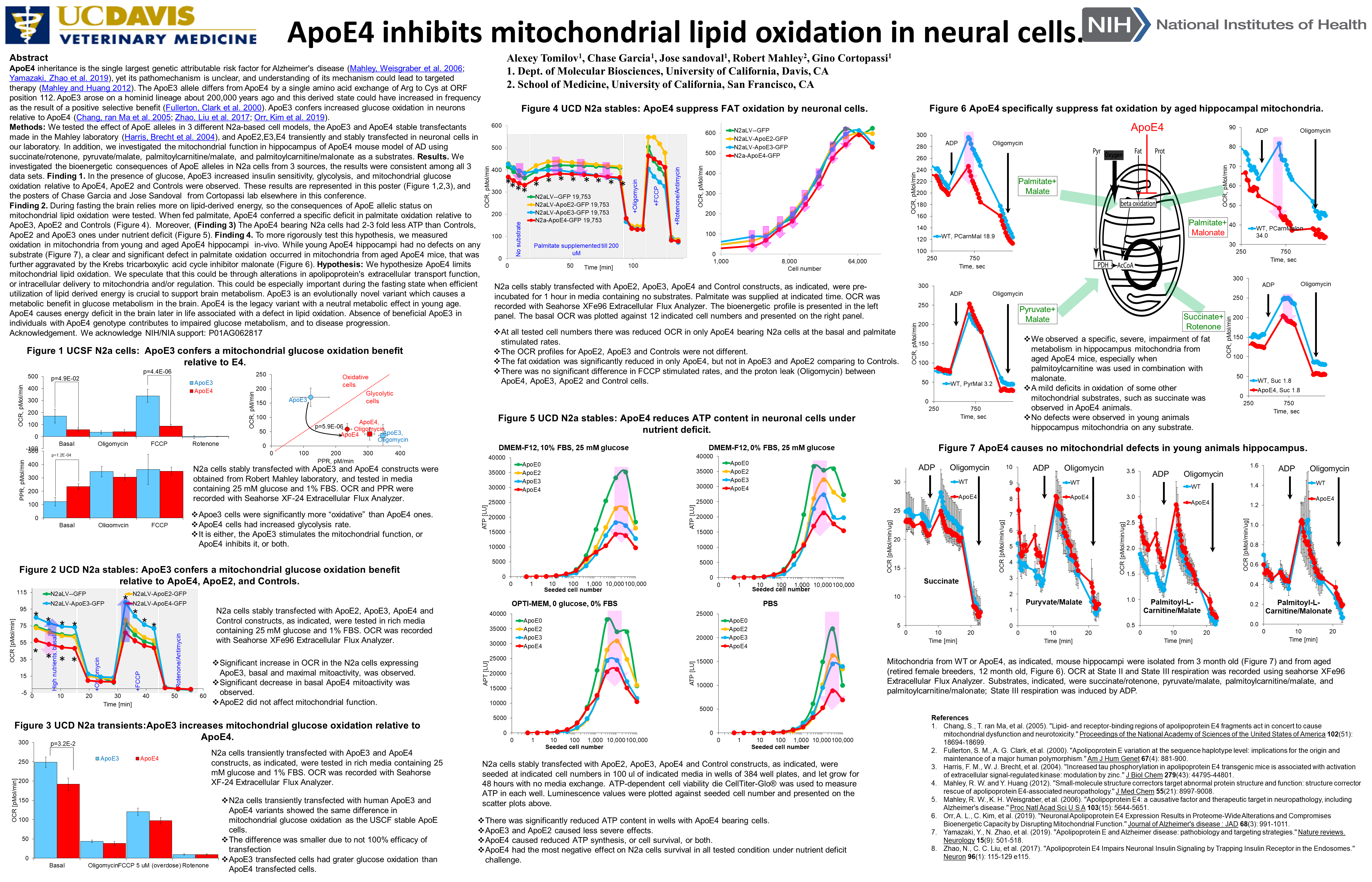
Finding 2. During fasting the brain relies more on lipid-derived energy, so the consequences of ApoE allelic status on mitochondrial lipid oxidation were tested. When fed palmitate, ApoE4 conferred a specific deficit in palmitate oxidation relative to ApoE3, ApoE2 and Controls (Figure 4).
Moreover, (Finding 3) The ApoE4 bearing N2a cells had 2-3 fold less ATP than Controls, ApoE2 and ApoE3 ones under nutrient deficit (Figure 5).
Finding 4. To more rigorously test this hypothesis, we measured oxidation in mitochondria from young and aged ApoE4 hippocampi in-vivo. While young ApoE4 hippocampi had no defects on any substrate (Figure 7), a clear and significant defect in palmitate oxidation occurred in mitochondria from aged ApoE4 mice, that was further aggravated by the Krebs tricarboxylic acid cycle inhibitor malonate (Figure 6).
Hypothesis: We hypothesize ApoE4 limits mitochondrial lipid oxidation. We speculate that this could be through alterations in apolipoprotein's extracellular transport function, or intracellular delivery to mitochondria and/or regulation. This could be especially important during the fasting state when efficient utilization of lipid derived energy is crucial to support brain metabolism. ApoE3 is an evolutionally novel variant which causes a metabolic benefit in glucose metabolism in the brain. ApoE4 is the legacy variant with a neutral metabolic effect in young age. ApoE4 causes energy deficit in the brain later in life associated with a defect in lipid oxidation. Absence of beneficial ApoE3 in individuals with ApoE4 genotype contributes to impaired glucose metabolism, and to disease progression.